Abstract
Introduction: Thrombopoietin-receptor agonists (TPO-RAs) are used in the treatment of chronic immune thrombocytopenia (ITP), a disorder characterized by prolonged low platelet counts (PCs) that pose a risk of serious bleeding episodes. The TPO-RAs eltrombopag (ELT) and romiplostim (ROMI) have been available for over 10 years. ELT is an oral medication with food restrictions and carries a boxed warning for hepatoxicity; additionally, ELT requires hepatic monitoring and has a potential need for statin dosing adjustment. ROMI, an injectable, is typically administered in a health care practitioner's office on a weekly basis without food restrictions. Avatrombopag (AVA), an oral medication taken with food, is the most recently approved TPO-RA for the treatment of chronic ITP (2019 by FDA and 2021 by EMA). AVA does not require hepatic monitoring or potential statin dose adjustment. A high proportion of patients (~90%) responded to AVA in clinical trials, and treatment was well-tolerated; however, limited real-world effectiveness data have been reported to date. This study is the first to use administrative claims linked to lab data to evaluate treatment response following the initiation of AVA in patients with ITP in the US.
Methods: This retrospective study used administrative claims data from the Komodo Healthcare Map (02/01/2017-02/28/2022) linked with platelet count (PC) laboratory data from Quest Diagnostics. Patients with >1 diagnosis of ITP (ICD-9-CM: 287.31; ICD-10-CM: D69.3) followed by ≥1 paid prescription for AVA (first such prescription was defined as the index date) and ≥1 PC during the twelve-month period following the index date (study observation period) were selected. The percentages of patients achieving clinically meaningful PC thresholds (≥30 x 109/L, ≥50 x 109/L, ≥75 x 109/L, and ≥100 x 109/L) were assessed at any time within 1 year of AVA initiation. Additionally, median and mean PCs were assessed at baseline (PC closest to and within 3 months before the index date), and within each 3-month period after AVA initiation, among the subgroups of patients with available PC data in each subsequent 3-month period.
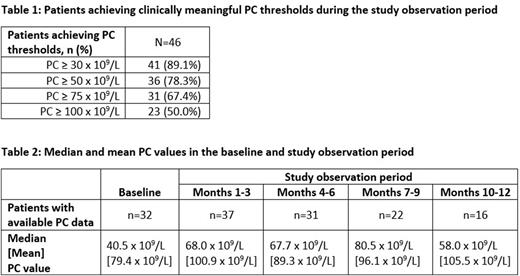
Results: A total of 46 patients met all eligibility criteria. On average, patients were 56.1 (±16.7) years of age, and 52.2% were female. Of the 46 patients, 32 (69.6%) had a baseline PC; among them, median baseline PC was 40.5 x 109/L. Across the full sample of 46 patients, 89.1%, 78.3%, 67.4%, and 50.0% of patients achieved thresholds of ≥30 x 109/L, ≥50 x 109/L, ≥75 x 109/L, and ≥100 x 109/L, respectively, during the 12-month period following AVA initiation (Table 1). Median PCs were 68.0 x 109/L during months 1-3 post initiation, 67.7 x 109/L between months 4-6, 80.5 x 109/L between months 7-9, and 58.0 x 109/L between months 10-12 (Table 2). Mean PCs were notably higher, reflecting the wide variability in the platelet counts data.
Conclusions: The majority of patients with ITP in this real-world study achieved clinically meaningful platelet counts following AVA initiation. Throughout the 12-month study observation period, mean and median PC values obtained were above a clinically meaningful threshold of 50 x 109/L. While this study was limited by the availability of PC data, the results are consistent with those from the pivotal clinical trials of AVA. Additional real-world studies are warranted to confirm these findings.
Disclosures
Oladapo:Sobi, Inc.: Current Employment. Kolodny:Sobi, Inc.: Current Employment. Vredenburg:Sobi, Inc.: Current Employment. Jamieson:Sobi, Inc.: Current Employment. Swallow:Analysis Group, Inc.: Current Employment. Goldschmidt:Analysis Group Inc.: Current Employment. Zichlin:Analysis Group, Inc.: Current Employment. Davidson:Analysis Group, Inc.: Current Employment. Yu:Analysis Group, Inc: Current Employment. Yee:Sobi, Inc.: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal